Resources
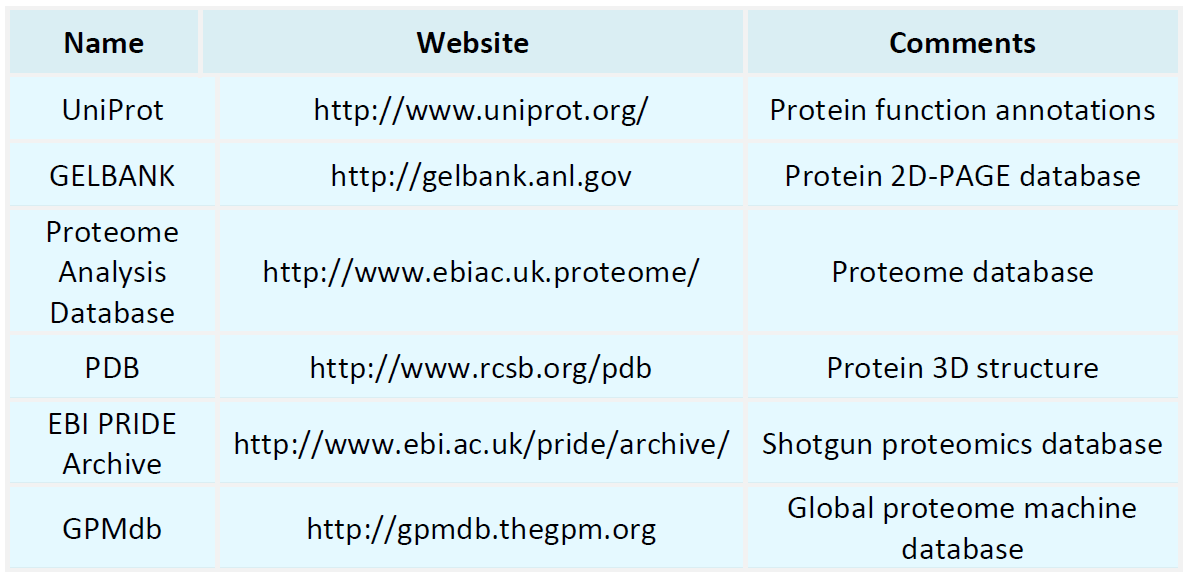
Proteomics Databases
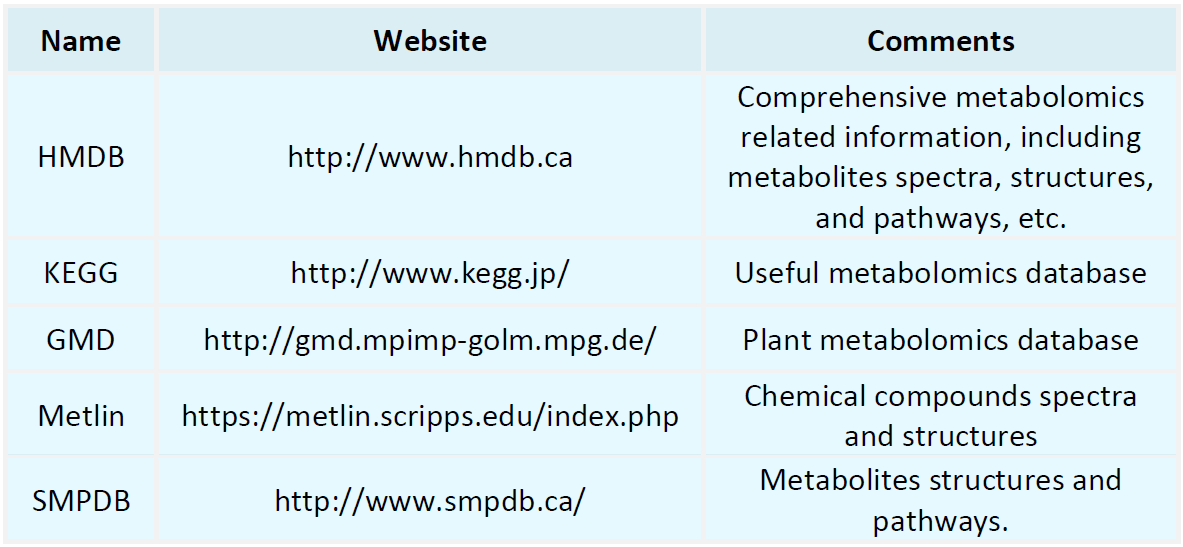
Metabolomics Databases
-
• Impurity Detection Based on SEC and RPLC Methods
In protein purity analysis, detecting impurities is crucial for assessing the quality of the sample. Reverse-phase high-performance liquid chromatography (RP-HPLC) and size exclusion chromatography (SEC) are widely used techniques, each offering specific advantages in detecting and separating impurities in protein samples.
-
• Detection and Analysis of Protein Post-Translational Modifications
Protein post-translational modifications (PTMs) are crucial for the regulation of cellular processes and the functional diversification of proteins. PTMs occur after protein biosynthesis, altering protein properties such as activity, localization, stability, and interactions. Understanding PTMs is essential for comprehending cellular mechanisms and disease pathogenesis.
-
• Quantitative Analysis of Low-Abundance Proteins Using DIA-PRM
In modern biological research, the quantitative analysis of low-abundance proteins has always been a challenge. These proteins are usually present at extremely low concentrations in biological systems, but they play crucial roles in various physiological processes, disease progression, and drug responses. As a result, accurately and efficiently quantifying these low-abundance proteins has become a hot and challenging topic in proteomics research.
-
• Analysis of Post-Translationally Modified Peptides Using LC-MS/MS
Proteins undergo various post-translational modifications (PTMs), which are crucial for regulating protein function and stability. These modifications, including phosphorylation, acetylation, ubiquitination, and others, add layers of functional diversity to the proteome. With the advancement of mass spectrometry, Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) has become an indispensable tool for the detailed study of PTMs.
-
• Detection and Analysis of Low-Abundance Proteins Using 4D Proteomics
The rapid advancement of proteomics has enabled scientists to explore protein networks within organisms in greater depth. However, detecting and analyzing low-abundance proteins remains a major challenge in proteomics research. These proteins often play pivotal roles in biological processes and disease states, making their study crucial for understanding underlying biological mechanisms. The advent of 4D proteomics technology offers new avenues for overcoming this challenge.
-
• Steps for Identifying PTM Sites via Nano-LC-MS/MS
In proteomics research, post-translational modifications (PTMs) play a pivotal role in regulating protein function. The identification and localization of these modification sites are critical for understanding protein function, cellular signaling, and disease mechanisms. Nano-LC-MS/MS technology, recognized for its high sensitivity and resolution, serves as a powerful tool for identifying PTM sites.
-
• Detection of Protein Sumoylation Using NanoLC-MS/MS
Protein SUMOylation is a post-translational modification where small ubiquitin-like modifier (SUMO) proteins are covalently attached to target proteins. This modification is crucial in regulating various cellular processes, including cell cycle control, gene expression, stress responses, and protein stability. Abnormal SUMOylation is closely linked to the onset and progression of various diseases, making the detection and analysis of SUMOylated proteins of significant scientific interest.
-
• Detection of Post-Translationally Modified Peptides by Mass Spectrometry
Post-Translational Modifications (PTMs) are critical for regulating protein functions, localization, stability, and interactions. Common PTMs include phosphorylation, acetylation, glycosylation, and ubiquitination. Detecting these modifications is vital for understanding cellular signaling pathways and disease mechanisms. Mass spectrometry (MS), with its high sensitivity and resolution, has emerged as the leading technique for studying PTM-modified peptides.
-
• MtoZ Biolabs High-Depth Plasma Proteomics Service (HD Blood Plus)
Plasma proteomics plays a crucial role in the discovery of biomarkers, disease mechanisms, and therapeutic targets due to the dynamic nature and diversity of plasma proteins. MtoZ Biolabs offers the High-Depth Plasma Proteomics service, which leverages cutting-edge mass spectrometry and data analysis platforms to achieve unprecedented depth and precision in plasma proteome profiling. This service is designed for researchers aiming to explore the complex plasma proteome with high sensitivity and accuracy,
-
• Explore Comprehensive Phosphoproteomics with DIA Technology at MtoZ Biolabs
At MtoZ Biolabs, we utilize cutting-edge Data-Independent Acquisition (DIA) technology combined with our extensive expertise in proteomics and mass spectrometry to offer a comprehensive phosphoproteomics analysis service. This service helps researchers uncover novel insights into phosphorylation events, signal transduction pathways, and the mechanisms behind various diseases. Our DIA-based phosphoproteomics workflow ensures high-quality and reliable results with unparalleled coverage. The process begins wit
How to order?







