C-Terminal Sequencing Service
Protein integrity is a crucial charateristics for protein, as protein truncation or microheterogeneity can lead to malfunction of protein. It is even more critical for the quality tests of biopharmaceutical products, such as antibodies and vaccines. Thus, analysis of the C-terminal sequence of protein products is necessary for evaluating the quality and function of protein products. MtoZ Biolabs have developed a LC-MS/MS system, which is suitable for analyzing both N-terminal sequence and C-terminal amino acid sequence and PTMs. We strictly follow the ICH Q6B Guidance to fulfill our customers' needs.
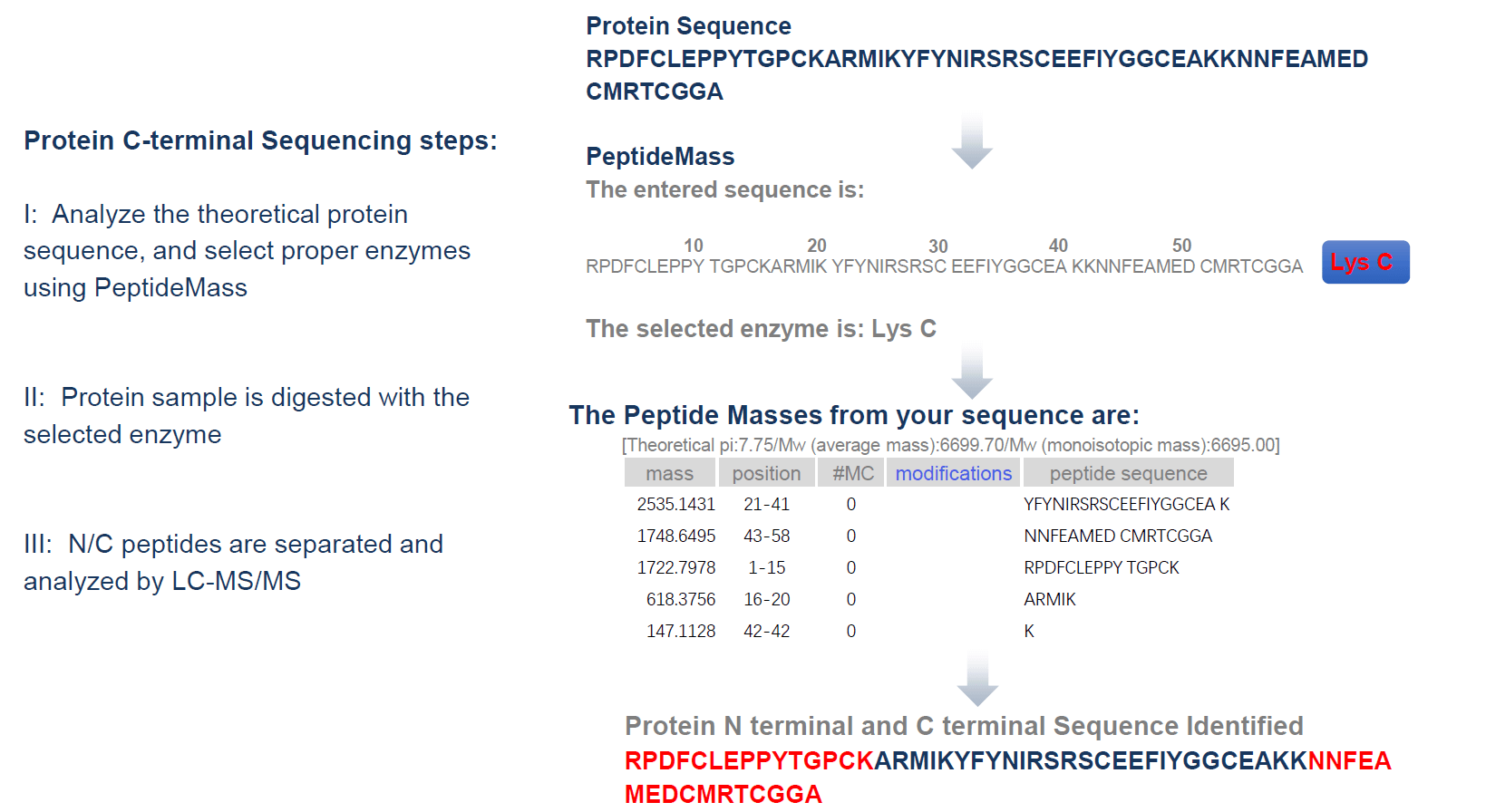
Analysis Workflow
Applications
1. Determination of N/C-Terminal Sequence of Proteins/Peptides/Antibodies/Vaccines
2. Analysis of PTMs
Sample Submission Requirements
| Format | Gel bands, gel spots, and liquid samples are acceptable. |
| Quantity | Gel bands/spots that are visible to naked eyes are adequate for protein identification. For liquid samples, A total of 5-10 ug proteins are required. |
| Purity | Purity of liquid samples should be as high as possible. For liquid sample <10 ug, please avoid large amounts of detergent and salt ions, and note the buffer composition and the estimated amount of total proteins when submitting samples. |
| Note | All reagents/solvent used must be of the highest purity to reduce contaminating substances. Samples should be handled with extreme caution and always in clean condition. Any source that may introduce contaminating proteins should be eliminated. |
Deliverables
1. Experimental Procedures
2. Parameters of Liquid Chromatography and Mass Spectrometer
3. MS Analysis Raw Data Files
4. N/C-Terminal Amino Acid Sequences
Related Services
Protein Sequencing
Protein Full-Length Sequencing
Protein Analysis
PTM Analysis
Submit Inquiry
How to order?







